According to MP DOCTOR(formerly Market Point) by KG Zeroin on the 11th, as the KOSPI index surged past 3,200 intraday and the KOSDAQ rebounded above 800, pharmaceutical and biotech stocks broadly rallied in line with the overall market strength.
|
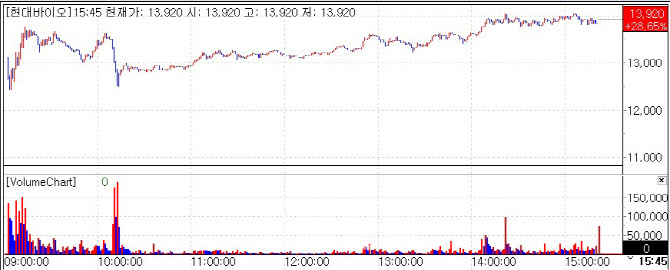
Among them, Hyundai Bioscience closed at 13,920 KRW, up 28.65%(3,100 KRW) from the previous day marking the highest percentage gain among pharmaceutical and biotech stocks. The rally was largely attributed to its announcement of a bonus issue the day prior.
Hyundai Bioscience announced it will issue one bonus share for every share held, amounting to a total of 48,019,352 new shares. The company’s total number of outstanding shares will rise to 96,038,704. The record date for the stock bonus is July 25, with the listing of the new shares expected on August 14.
The stock has risen in eight out of the past nine trading days since July 1. Aside from the stock bonus, investor sentiment appears to be buoyed by the company’s progress in dengue fever clinical trials in Vietnam. Hyundai Bioscience signed a memorandum of understanding with major Vietnamese hospitals to collaborate on Phase 2/3 trials targeting 210 PCR confirmed dengue patients. The combined Phase 2/3 trial is expected to begin soon, with plans to seek Emergency Use Authorization(EUA) from the World Health Organization(WHO) and ASEAN countries if successful.
Additionally, the company’s preclinical results showing that it scientifically demonstrated “pseudo-resistance” in pancreatic cancer using the world’s first human-derived organoid model conducted jointly with its subsidiary Hyundai ADM also supported the rally. The company plans to officially present the findings on July 21.
“This announcement will correct the longstanding misconception about cancer resistance, which has been blamed for 80 years of failed cancer treatments,” said a Hyundai Bioscience official. “It’s a turning point that identifies metastatic cancer which accounts for 90% of the 10 million annual cancer deaths for what it truly is.”
HLB Life Science Surges on NDA Plans for Bile Duct Cancer Drug
Shares of HLB affiliates rose notably on the day. HLB Life Science gained 10.52%(490 KRW) to close at 5,150 KRW. Parent company HLB rose 1.19% to 51,200 KRW, while HLB Panagene gained 2.65% to 2,130 KRW. HLB Science, listed on KONEX, jumped 14.98% to 1,412 KRW. In total, all 11 HLB-related stocks closed higher.
The rally followed HLB’s disclosure of its drug development plans. The company announced it plans to submit an NDA for its targeted bile duct cancer therapy, rilapugratinib(RLY-4008), by year-end or early next year, and expects FDA approval by summer 2026.
According to HLB, the NDA preparations?led by its subsidiary Elevar include compiling clinical data, manufacturing data, and GMP inspection readiness for both active pharmaceutical ingredients and finished products. The company is planning a pre-NDA meeting with the FDA later this year, after which a firm submission timeline will be finalized.
HLB emphasized that rilapugratinib has been designated an “innovative drug,” increasing the likelihood of priority review, which could shorten the FDA’s review period from 10 to 6 months.
Addressing concerns about potential CMC(Chemistry, Manufacturing, and Controls) issues like those seen in the case of rivoceranib?the company stated: “Rilapugratinib is a small-molecule drug, and small molecules generally offer greater production stability and quality control compared to biologics.” The company added that its API manufacturer, Piramal Healthcare Canada, had passed several previous FDA inspections, including a recent one in May, and had also cleared audits by other major regulatory authorities such as Japan.
HLB also revealed plans to initiate a tumor-agnostic global clinical trial within the year, seeking broader indication approvals for rilapugratinib beyond FGFR2 fusion-positive bile duct cancer.
Onconic Therapeutics Soars for Fourth Straight Session
Onconic Therapeutics jumped 16.49%(4,000 KRW) to 28,250 KRW, with after-hours trading pushing shares close to 30,000 KRW a gain of nearly 23%. The company has posted gains for four consecutive trading sessions.
However, Onconic stated that it is unaware of any specific catalysts behind the rally. “There hasn’t been a particular event driving the surge,” a company spokesperson said. “But recent investor optimism may be tied to the company’s submission of a protocol amendment for a Phase 3 trial of its GERD treatment Zacubo targeting non-erosive reflux disease(NERD), as well as nearing the completion of a Phase 3 trial in China.”
According to Onconic, the China Phase 3 trial is expected to conclude in the second half of 2025, after which results will be released. The company anticipates royalty revenues and product sales following commercialization in China.