|
Regarding the South Korean Phase 3 trial, Bio Solution highlighted MRI images taken at 24, 48, and 96 weeks, demonstrating CartiLife’s consistent efficacy compared to microfracture surgery. The P-value, which measures statistical significance, was recorded at 0.0131, well below the commonly accepted threshold of 0.05. Additionally, KOOS scores, which assess pain and mobility, showed continuous improvement over 24, 48, and 96 weeks. The cartilage regeneration effect was observed not only in younger patients but also in individuals over 50 and those with osteoarthritis.
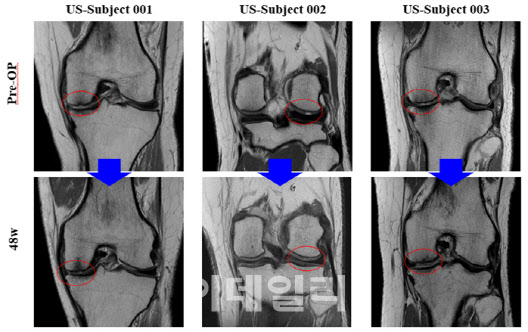
For the U.S. Phase 2 trial, Bio Solution released MRI images from select patients, visually confirming cartilage regeneration in defect areas up to 48 weeks post-procedure. Key evaluation metrics, including KOOS (pain and mobility improvement), VAS, and IKDC scores, also demonstrated consistent improvement over 24 and 48 weeks.
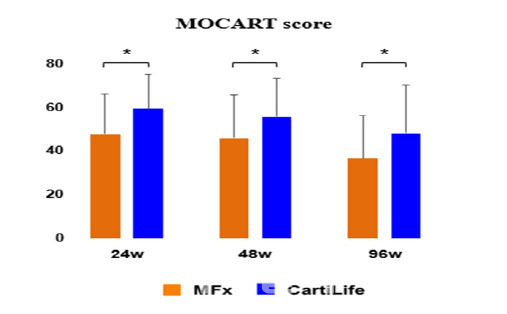
Previously, Bio Solution reported that the MOCART score at 48 and 96 weeks post-surgery in the South Korean Phase 3 trial showed statistically significant improvement compared to the active control group (microfracture surgery). KOOS total score changes at these time points also confirmed non-inferiority to the control group. Secondary efficacy indicators, such as IKDC score (assessing knee function and activity) and KOOS score (measuring pain and daily activity performance), showed statistically significant differences, with safety also confirmed.
“This conference allowed us to highlight CartiLife’s superior cartilage regeneration capabilities and expand our network with interested pharmaceutical companies,” said Bio Solution CEO Lee Jung-sun. “Following the AAOS, we have continued meetings to strengthen partnerships. We will do our utmost to ensure that this and future global conferences lead to successful license-out agreements for CartiLife.”
|