Shares of Green Life Science, Sugentech and Cellid all rose, supported by signs of a possible COVID-19 resurgence even as most sectors declined. Green Life Science climbed 8.2% to 2,760 won, Sugentech gained 6.6% to 7,250 won, and Cellid advanced 5.1% to 4,190 won, according to KG Zeroin’s MP Doctor data.
|
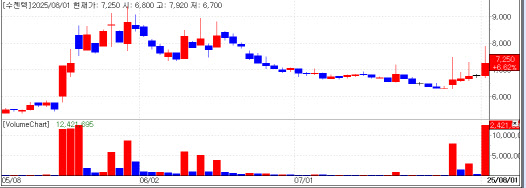
Sugentech has surged for six consecutive sessions, rising 14.9% since July 25. The company makes SGTi flex COVID-19 & Flu A/B, a rapid test kit that simultaneously detects COVID-19 and influenza A/B with color-coded results for improved accuracy and readability.
Sugentech won export approval from the Ministry of Food and Drug Safety in 2022 and became the first Korean company to obtain CE CoC certification for COVID-19 self-testing in Europe. Its kits are exported to 50 countries.
“Sugentech is regaining market attention because of the cash it amassed during the pandemic and its continued push into new diagnostics, including women’s hormone testing,” an industry official said.
Green Life Science, a maker of pharmaceutical intermediates and raw materials, also benefited from COVID-linked sentiment. The company is widely known as the exclusive supplier of a key intermediate for Pfizer’s oral antiviral Paxlovid, although it does not disclose end-use details.
The stock has tended to move in line with pandemic headlines, despite limited earnings catalysts.
|
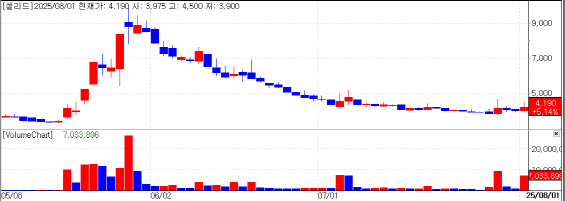
Cellid’s advance was fueled by the European patent registration of its adenovirus vector platform used in its COVID-19 vaccine candidate, AdCLD-CoV19-1 OMI.
The patent, already granted in Korea, the United States, China, Russia, and Japan, addresses the risk of unintentional replication competent adenovirus formation during vaccine production. Cellid says the technology offers a cost efficient solution compared with the high-cost strategies employed by global rivals, such as engineering production cell lines or using alternative adenovirus serotypes.
“The European registration affirms our proprietary platform’s novelty and global recognition,” CEO Changyul Kang said. “It is a core technology for safe, high quality vaccines and a foundation for Korea’s domestic response to future infectious diseases.”
The vaccine has completed Phase 3 dosing and is in follow-up monitoring.
COVID-19 hospitalizations have risen for four consecutive weeks. The Korea Disease Control and Prevention Agency reported 139 inpatients nationwide in the 30th week of 2025 (July 20) up from 123 the previous week. Authorities are preparing additional countermeasures in August if the trend continues.




![[조진웅 논란] 기업 띄우려다 날벼락 맞는 ‘연예인 리스크'](https://image.edaily.co.kr/images/vision/files/NP/S/2025/12/PS25121000744t.jpg)



