Among them, the corporate value of AimedBio which is writing a new history in the KOSDAQ market and Hyundai Pharm which is set to expand its influence in the hair loss treatment market are evaluated as having significant potential for further growth.
|
AimedBio Surpasses 3 Trillion Won Market Cap Just Two Trading Days After KOSDAQ Listing
According to KG Zeroin MP DORTOR (formerly MarketPoint), on this day, AimedBio, Hyundai Pharm and Natural Endotech were listed among the domestic stock market's 'Top 10 Gainers' in the Bio segment.
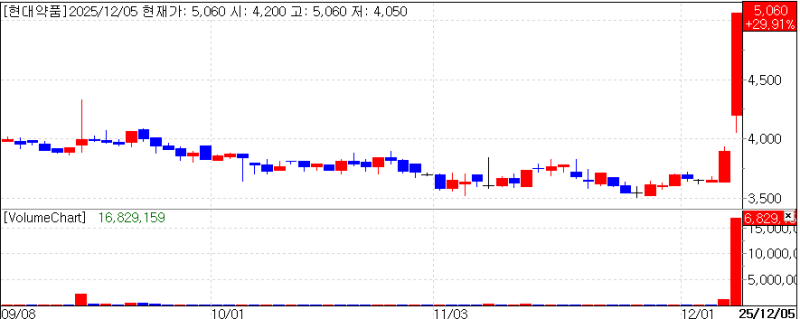
All three companies effectively recorded the upper trading limit. Their stock prices surged by 30.00% (closing at 57,200 won), 29.91% (5,060 won), and 29.83% (3,090 won) respectively, compared to the previous day.
Although all were upper-limit gains, their significance differed. The company that garnered the most attention was AimedBio which surpassed a 3 trillion won market capitalization and ranked 17th in market cap just two days after its KOSDAQ debut.
AimedBio closed the previous day at 44,000 KRW, a 300.0% increase from its initial public offering (IPO) price of 11,000 won achieving the 'Quadruple' (four times the IPO price). On the 5th, its second trading day it also closed at the upper limit pushing its market cap up to 3.6697 trillion won.
A major factor behind AimedBio's explosive corporate value surge is the creation of substantial results in Antibody-Drug Conjugate (ADC) new drugs. AimedBio has successfully licensed out its bladder cancer treatment candidate 'AMB302' to Biohaven in the US and its highly expressed solid tumor treatment candidate 'ODS025' to Boehringer Ingelheim in Germany.
Including these deals it has achieved cumulative technology transfer success of over 3 trillion won even in its unlisted phase. Its future remains bright with additional technology transfer possibilities. Key pipelines include two bispecific ADC solid tumor treatments and AMB001 which is being developed as a treatment for atopic dermatitis and dementia.
AimedBio is also pursuing the re-licensing of 'AMB303,' a treatment targeting ROR1-overexpressed solid tumors, co-developed with SK Plasma. Furthermore, it is a co development partner in Samsung Biologics' 'ADC Toolbox Program.' The funds secured through the listing are planned for investment into non clinical trials for subsequent pipelines (PP1, PP2, PP3) and the development of new platforms.
The biggest challenge for a biotech company achieving a turnaround to profitability is predicted to happen in its listing year. Consecutive technology exports have enabled the company to achieve an operating profit surplus in the second half of last year and the first half of this year leading to the goal of achieving its first annual net profit this year.
AimedBio targets maintaining profitability by achieving over 27 billion won and 40 billion won in revenue solely from technology transfers in 2026 and 2027, respectively.
The growth potential of AimedBio is also trusted by influential domestic pharmaceutical and biotech companies, which is demonstrated by its Strategic Investors (SIs). The Samsung Life Science Fund jointly invested by Samsung C&T, Samsung Biologics and Samsung Bioepis along with Yuhan Corporation volunteered as early backers by participating in AimedBio's initial investment.
Huh Nam goo, CEO of AimedBio emphasized "We will become a next generation biotech company that proves new standards with performance"
adding "Based on this we will strive to deliver new drugs to more patients."
|
High Expectations Continue for Hyundai Pharm, While Natural Endotech Faces Higher Uncertainty
Hyundai Pharm also has a high probability of further increases in corporate value, driven by the continuation of favorable events. This includes the sustained impact of the successful Phase 3 clinical trial of the male pattern hair loss new drug 'Clascoterone 5% Solution' by global pharmaceutical and biotech firm Cosmo Pharmaceuticals.
Hyundai Pharm holds the exclusive domestic development, approval, and sales license for the acne treatment 'Winlevi' from Cassiopea, a subsidiary of Cosmo Pharmaceuticals. Having obtained product approval for Winlevi from the Ministry of Food and Drug Safety in September, the company is preparing for its domestic launch.
The industry anticipates that the successful commercialization of Winlevi will lead to the development, approval, and exclusive sales license for Clascoterone 5% Solution. The fact that the active ingredient in Winlevi is based on Clascoterone also supports this industry speculation.
Winlevi is a treatment that uses a 1% concentration of Clascoterone. Clascoterone 5% Solution is expected to bring about a paradigm shift in the hair loss treatment market, having proven its potential in clinical trials.
Cosmo Pharmaceuticals recently announced that it met the primary endpoints in both Phase 3 trials involving 1,465 male pattern hair loss patients across 50 regions in the US and Europe. According to the Clascoterone 5% Solution clinical results the target hair count increased by 539% compared to the placebo, with another trial showing a 168% improvement effect.
Consequently Clascoterone 5% Solution is being hailed as a new mechanism candidate to emerge in male pattern hair loss in about 30 years.
Furthermore the company is shedding the image of a traditional pharmaceutical company, typically represented by over the counter (OTC) drugs like 'Minoxidil Solution' and 'Bumulli' (an insect bite cream). This is thanks to its expansion of influence as a new drug developer, backed by stable profitability.
Hyundai Pharm is conducting a Phase 2b clinical trial for the diabetes treatment candidate 'HDNO-1605.' Other promising pipelines with high commercialization potential include the hyperlipidemia treatment 'BSDO-2301' and three improved compound drugs for circulatory diseases.
Hong Soon jae CEO of Biobook stated "Hyundai Pharm which possesses both a stable revenue structure and technology transfer potential is one of the attractive investment options that differ from the high risk high return segment of the biotech sector."
However he advised caution noting, "Investors should be mindful that the current increase in corporate value is reliant on external issues."
In contrast to AimedBio and Hyundai Pharm, there is strong expert advice to be cautious about further investment in Natural Endotech. The prevailing view is that its stock price frequently fluctuates without clear reason potentially trapping investors momentarily. On the 5th, its stock also rose without any distinct news.
Natural Endotech, which was severely impacted by the 'Fake Baeksuo' controversy in 2015, partially recovered its reputation with a Supreme Court ruling last June. However the damage caused by the litigation has yet to be fully recovered.
CEO Hong remarked "Natural Endotech frequently shows unpredictable stock movements, making the risk high for long term investment."
adding "As the overall market is in a bull run this is the time to focus on blue chip stocks."