Survival Benefit Even in High-Risk Liver Cancer Patients
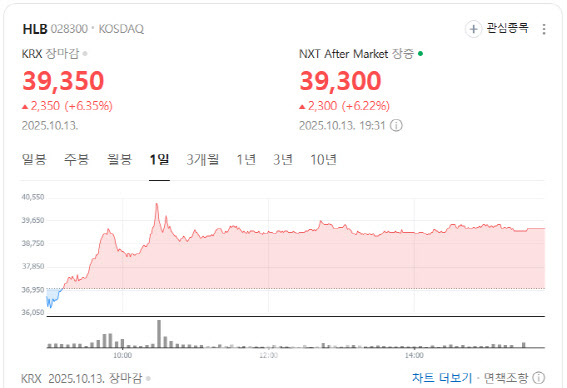
According to MP Doctor (formerly MarketPoint) by KG Zeroin, HLB closed at 39,350 won, up 6.35% from the previous session.The company announced that its liver cancer therapy Rivoceranib, in combination with Camrelizumab from China’s Jiangsu Hengrui Medicine, significantly extended survival in patients with the poorest prognosis.
|
HLB’s U.S. subsidiary, Elevar Therapeutics, will officially present the data at the European Society for Medical Oncology (ESMO) 2025 meeting in Berlin on October 17 (local time). According to the released abstract, the Rivoceranib-Camrelizumab combination improved both median overall survival (mOS) and median progression-free survival (mPFS) in unresectable hepatocellular carcinoma (HCC) patients with extrahepatic spread (EHS) or macrovascular invasion (MVI)-the most challenging subgroups to treat.
Patients with extrahepatic spread had a median OS of 23.5 months, more than 10 months longer than those treated with sorafenib (13.0 months). Among patients with macrovascular invasion, mPFS improved from 3.0 months to 5.5 months.
“Rivo+Camrel’s combination showed a clinically meaningful survival benefit while maintaining manageable safety even in high-risk patients,” said Han Yong-hae, HLB Group’s Chief Technology Officer. “These data strengthen its position as a potential first-line therapy for advanced liver cancer.”
U.S. ‘Biosecurity Act’ Nears Final Passage
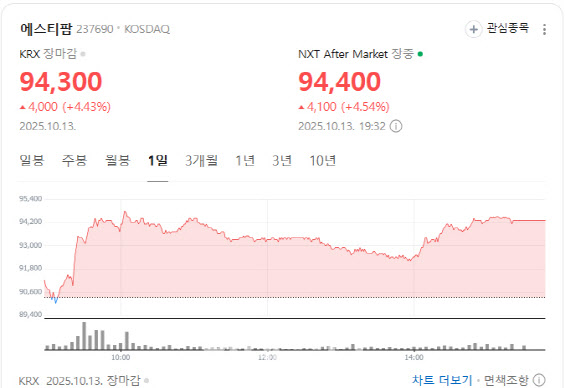
ST Pharm rose 4.43% to close at 94,300 won, as investors bet that the passage of the U.S. BIOSECURE Act in the Senate would benefit Korean CDMO companies by restricting Chinese rivals’ access to the American market.
|
If the bill passes the House-Senate reconciliation process and receives presidential approval, it could take effect by year-end. Analysts say the law will significantly weaken Chinese biotech players’ influence in the U.S. market.
While giants like Samsung Biologics may absorb much of the large-scale demand, ST Pharm is expected to see more immediate benefits among smaller biotech clients. Roughly 80% of U.S. small and mid-sized biotechs currently rely on Chinese CDMOs, creating potential for partner realignment in favor of Korean firms.
However, competition from Japan and India is also intensifying. Japan’s Fujifilm recently completed the largest cell culture biologics manufacturing facility in North Carolina, and Indian firms are rapidly expanding their footprint through low-cost, high-volume production.
CEO Exit Raises Concerns Over Pipeline Continuity
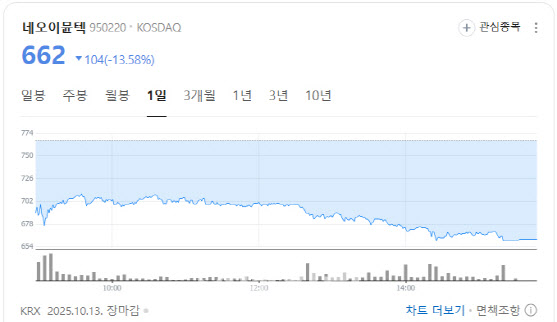
NeoImmuneTech fell 13.58% to 662 won after announcing the resignation of CEO Oh Yoon-seok on October 10. The board appointed Kim Tae-woo, Chief Financial Officer, as acting CEO.
|
Before joining NeoImmuneTech, Oh worked for over six years at the FDA’s Division of Clinical and Immunology Pharmacology, where he oversaw drug review, clinical trial design assessments, and immuno-oncology evaluations. Leveraging that experience, he had led FDA discussions and coordination with the Biomedical Advanced Research and Development Authority (BARDA) for the company’s flagship Acute Radiation Syndrome (ARS) therapy, targeted for commercialization in 2026.
The ARS therapy is based on NT-I7 (efineptakin alfa), NeoImmuneTech’s proprietary long-acting T-cell-enhancing immunotherapeutic platform, designed to restore immune cell counts damaged by radiation exposure. The candidate is currently in nonhuman primate testing.
In August, interim data showed that NT-I7 increased survival rates by 43 percentage points over control groups in irradiated primates. Surviving subjects also exhibited higher absolute lymphocyte counts (ALC), suggesting a strong immune recovery effect. NT-I7 was originally licensed from Genexine in 2015 for $12.5 million and remains the core of NeoImmuneTech’s immunotherapy pipeline.
A company spokesperson said, “Both the ARS and CAR-T combination trials are being managed by our U.S. research team, so there are no disruptions to development,” adding that “the CEO’s resignation is unrelated to pipeline performance or progress.”