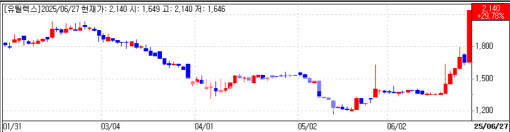
Key drivers behind Eutilex sudden surge
Eutilex Co. hit its 30 percent daily trading limit, closing at 2,140 won, up 491 won, valuing the cell-therapy developer at 78.8 billion won ($58 million). Investors piled in ahead of an interim Phase 1 readout for its CAR-T therapy EU307, scheduled for next month at the Asia-Pacific Primary Liver Cancer Expert meeting (APPLE) in Kobe, Japan.
PharmEdaily first reported the pending data release in a June 22 pay-to-read article titled “Eutilex’s Solid-Tumor CAR-T to Show Interim Data in Japan Next Month,” which was later distributed free on major portals. The report, traders said, helped fan anticipation of positive results.
|
Eutilex obtained Korean Phase 1 clearance in 2023 for an open-label study of up to 12 patients with relapsed or refractory liver cancer. About half the cohort has been dosed. Yonsei University’s Prof. Kim Do-young will present safety and early efficacy findings in Kobe, the company said.
“APPLE will showcase EU307’s potential,” CEO Yoo Yeon-ho said, noting that Eutilex has strengthened its R&D bench with industry-seasoned leaders.
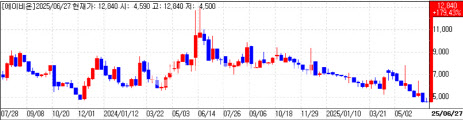
Abion’s antibody deal electrifies trading
Abion Co. also closed at its daily ceiling for a third straight session, ending 30 percent higher at 9,880 won. The oncology firm disclosed this week a license-out agreement for ABN501 an antibody targeting claudin-3 and four other proteins with an undisclosed partner.
Total deal value could reach $1.32 billion, including an upfront payment of $25 million and development milestones of up to $290 million. Commercial milestones may add another $1 billion.
|
“This partnership is a pivotal marker in Genecurix’s global expansion, following our recent commercialization pact in Japan with Hitachi High-Tech,” CEO Cho Sang-rae said, pledging further collaborations to drive growth.



!['과대망상'이 부른 비극…어린 두 아들 목 졸라 살해한 母[그해 오늘]](https://image.edaily.co.kr/images/vision/files/NP/S/2026/02/PS26021700001t.jpg)
